The establishment of an “mRNA hub” in South Africa to build the capacity of low- and middle-income countries (LMICs) to develop vaccines during the COVID-19 pandemic was widely hailed as a solution to Africa’s lack of manufacturing ability.
But three years after its launch in June 2021, the hub faces uncertainties, risks and shortfalls – including that it may simply become a “technological solution” in the mould of the current biopharmaceutical industry rather than a genuine transfer of knowledge and capacity to LMICs, according to a recent report
Authors Professor Matthew Herder, chair in Applied Public Health at Dalhousie University in Canada and Ximena Benavides from Yale University in the US, base their observations on interviews with 35 key players and numerous documents, some of which were obtained via an access to information request to the Canadian government.
The hub is the initiative of the World Health Organization (WHO) and the Medicines Patent Pool (MPP). Spurred by the failure of high-income countries to share their COVID-19 vaccines at a time of extreme need, the WHO and MPP selected a South African consortium comprising biopharmaceutical company Afrigen Biologics, vaccine producer Biovac and the SA Medicines and Research Council, as its partner to kickstart a facility capable of developing and producing mRNA vaccines in a LMIC.
Once this was done, they were to teach other facilities in LMICs across the world how to do the same.
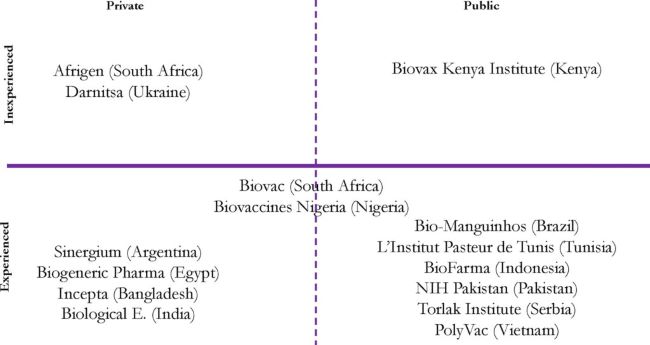
The mRNA programme currently includes the South African consortium and 14 other LMIC-based partners.
Championing voluntary IP transfer
But how the hub is governed and operates does not sufficiently transfer power and capacity to LMICs, the authors contend.
“The architects of the programme are working within the existing system of biopharmaceutical production and, at the same time, preserving their control over the programme’s design and preferred measures meant to remedy shortfalls inequitable access to mRNA-based interventions,” they argue.
“In particular, MPP continues to champion voluntary [intellectual property] licensing as the optimal means to improve local production capacity in LMICs even though that mechanism did not attract collaboration from more established mRNA manufacturers in the context of COVID-19 and slowed adoption of a more transformative end-to-end approach to R&D and manufacturing.”
They also argue that the “technological outcomes” of the programme are uncertain unless there is “significant reform and concerted effort to redistribute not just IP, but agency to LMIC actors”.
Without these “there is a significant risk that the programme, which is claimed by WHO and MPP as a collective effort to improve manufacturing capacity in LMICs for LMICs, will not solve the problem of equitable access to biopharmaceutical innovation”.
‘In line with status quo’
While the mRNA programme may improve the sharing of knowledge, the authors observe, it has been developed “in line with the status quo” of global biopharmaceutical production.
This includes “weak conditionalities around product affordability, participants’ freedom to contract with third parties, and acceptance of market-based competition”, they argue.
The WHO and MPP also exert “tight control over the programme” and this “evokes the dynamics that are often in play in global health, to the detriment of empowering LMIC-based manufacturers to generate mRNA products in response to local health needs”, they argue.
For example, the MPP has created its own technology transfer unit to manage technology transfer within the mRNA programme. But typically, technology is transferred from one party with direct experience in using it, to another, through sharing hands-on know-how.
“I’ve worked for more than 30 years in the industry. You do not have a remote group that does tech transfer. If a group is going to do tech transfer, it needs to be in the facility that’s sending the technology out,” one participant told the authors.
The hub’s donors – France, the European Commission, Germany, Norway, Belgium, Canada, South Africa and the African Union – have committed $117 million to the programme (with $89 million received so far).
But some of the high-income countries (HICs) that have invested in the mRNA programme have also made demands that have shaped the programme. Canada, for example, stipulated that its funding be allocated to the hub in Cape Town and four other countries only: Senegal, Nigeria, Kenya and Bangladesh.
“According to one interview participant, while HICs are supportive of transferring technology to LMICs, they would prefer that such transfers do not extend to the more upstream inputs into mRNA vaccine production, including novel LNPs and antigens,” the authors note.

Photo by Rodger Bosch for MPP
Contradictory legal agreements – and pricing silence
The report notes that the MPP has crafted a set of legal agreements including a technology transfer template in which LMICs are granted a “non-exclusive, royalty-free, non-sublicensable, non-transferable, irrevocable, fully paid-up, royalty-free licence” to the technology.
They also get access to any rights held by Afrigen and Biovac “to make or have made, use, offer for sale, sell, have sold, export or import” products in their respective territories and other LMICs.
In exchange, LMICs must grant MPP a worldwide, non-exclusive, royalty-free licence to data and inventions to “facilitate the development and equitable access of health technologies”.
Brazil’s Bio-Manguinhos has baulked at the idea that technology it has developed with funding from the Brazilian government “would flow to manufacturers from participating LMICs, which in some cases, are for-profit commercial entities, without anything in return”, with official Patricia Neves describing this as an “injustice”.
South Africa also contested the absence of royalties, and its agreement with MPP states that any licence may include a “royalty sacrifice”.
Meanwhile, Indonesia’s BioFarma negotiated the right to sell products to HIC.
“MPP also stopped short of requiring that resulting mRNA products be priced affordably for populations in need outside of a Public Health Emergency of International Concern (PHEIC),” the authors note.
If an mRNA product developed by an LMIC partner targets a PHEIC, they cannot charge more than the cost of production plus a 20% mark-up.
“Traditionally, MPP has not interfered in pricing. Our model is based on competition, and clearly, we are potentially giving this to 15 companies around the world,” MPP executive director Charles Gore told the authors.
In response, the authors remark that the MPP “appears to be comfortable relying on free-market competition among LMIC-based manufacturers instead of imposing affordability clauses when it comes to products generated by participating in the mRNA programme”.
Moving beyond COVID

It took Prof Petro Terblanche’s Afrigen only two months to develop an mRNA vaccine for COVID-19 based largely on a Moderna “recipe” published online. It has since transferred this knowledge to facilities in countries including Bangladesh, Serbia and Brazil.
But the urgency related to COVID-19 has passed. Expanding the programme’s focus upstream is now seen as crucial to its overall sustainability given that demand for COVID-19 vaccines is limited.
At a meeting in Bangkok in late 2023, WHO and MPP officials outlined “potential sub-consortia – engaging partners both inside and outside of the programme – focused on R&D around pathogens of shared, regional interest,” according to the authors.
Afrigen is increasingly focusing on the development of second-generation technologies important in mRNA production, such as novel lipid nanoparticles (LNPs), and new disease targets like TB, malaria and HIV.
“The critical question is whether the funding that has been secured for the programme and supporting the development of these second-generation mRNA technologies has been leveraged into a shared set of commitments geared towards improving equitable access,” the authors note.
While Afrigen targeted 11 potential diseases for mRNA product development, its proposals focusing on Lassa fever, RSV, and other disease targets have been turned down by a variety of funders.
Terblanche has conceded that her “hand will now be forced to prioritise” in favour of market rewards – particularly as the MPP expects the programme to be “self-sustaining” by 2026.
But an increasing number of “use patents” that claim IP on the use of mRNA technology are being filed in South Africa and other LMICs, and these threaten to block Afrigen and partners’ R&D plans.
Recalling that some of the companies that took part in the the influenza vaccine hub later shut down production, WHO’s Martin Friede estimates that if a handful of LMIC manufacturers manage to make mRNA vaccines, the programme will be an overall success.
Meanwhile, MPP’s Gore noted that “we are funding [Afrigen] to develop and then shift [the technology] out,” but “they don’t [yet] have a business model.”
MPP’s Marie-Paule Kieny speculates that Afrigen will, in the end, probably yield to market forces: “The hub is really there to establish a first platform and improvement, and to help with an early pipeline. After that we are fully aware that Afrigen is a private company, at one point they will try to find somebody to buy them out and to get the benefit,” she told the authors.
‘Failure of imagination’
But the authors describe the “near inevitability of Afrigen’s exit in the eyes of those who designed the programme” as an indication of the “underlying failure of imagination concerning how the mRNA programme is governed”.
A more “inclusive and decentralised” governance structure could “shield initiatives such as the mRNA programme from the risks and constraints posed by dominant market actors”.
This decentralized structure could involve “representatives from participating LMICs capable of steering the programme’s R&D towards local population” in the overall governance and day-to-day decision-making., they argue.
“Second, multiple actors would need to serve as regional mRNA hubs – as originally planned – to mitigate the risk that one organization’s failure (or acquisition by an outside actor) might compromise the programme as a whole,” they argue.
“Instead, WHO and MPP internalised programme decision-making within two hand-picked committees [the “Scientific and Technical Review Committee and the mRNA Scientific Advisory Committee], leaned on private actors like Afrigen to play crucial roles, preserved their discretion about what projects and partnerships to pursue, and limited input from LMIC governments and civil society during the programme’s first two-plus years of operation,” they note.
“It remains to be seen whether MPP, which has ascended in the sphere of global health during the pandemic as a result of its role as the central power broker for the entire mRNA programme, will over time cede some of its control and take the steps necessary to truly empower LMIC manufacturers,” they observe.
Image Credits: WHO, WHO, Kerry Cullinan.


![[VIEWPOINT] WHO’s mRNA vaccine hub faces uncertainty, structural weaknesses mRNA vaccine hub](https://ashenewsdaily.com/wp-content/uploads/2024/10/mRNA-vaccine-hub.jpg)