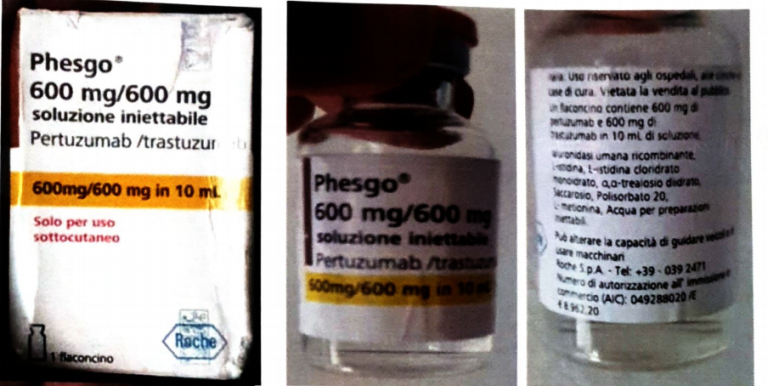
The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a public alert about a suspected counterfeit batch of Phesgo® 600mg/600mg/10ml, a breast cancer treatment injection.
NAFDAC said that the counterfeit batch, labeled C5290S20, was flagged by a doctor at Lagos University Teaching Hospital (LUTH-NSIA) after it was brought in by a patient for administration.
The agency stated that the Marketing Authorization Holder (MAH) for the drug, Roche, confirmed that the suspected counterfeit product does not match genuine materials.
This is due to key discrepancies among which includes non-existent batch number in Roche’s database, inconsistent language usage on the packaging, incorrect dates and tamper-evidence labels as well as missing anti-counterfeiting features.
“Although no physical sample was returned for chemical analysis, photographic evidence and expert examination confirmed the product’s falsified status.
“Phesgo 600mg/600mg is a life-saving breast cancer medication designed to kill cancer cells and prevent their growth. The presence of counterfeit versions poses severe health risks, as such products lack the regulatory safeguards to ensure safety, quality, and effectiveness.”
NAFDAC therefore directed all zonal directors and state coordinators to intensify surveillance and remove any counterfeit products from circulation.
The agency has also urged importers, distributors, retailers, and healthcare providers to procure medicines exclusively from licensed suppliers and verify their authenticity.