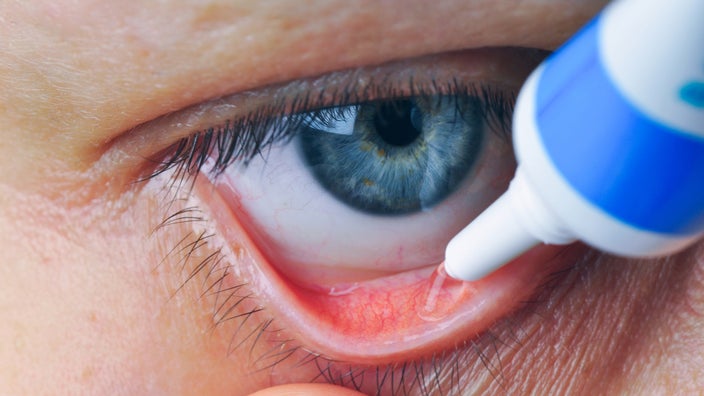
The FDA has approved VIZZ (aceclidine ophthalmic solution) 1.44% as the first and only eye drop treatment specifically for presbyopia in adults. Presbyopia, which causes blurry near vision due to aging, affects about 128 million adults in the US alone.
VIZZ is a once-daily prescription eye drop that works by contracting the iris sphincter muscle to create a pinhole effect. This mechanism improves near vision for up to 10 hours without causing distance vision blurring or a myopic shift. It starts working within 30 minutes of application.
Clinical trials involving hundreds of participants showed significant improvement in near vision and confirmed the treatment is well-tolerated. Side effects were mild and transient, including eye irritation, dim vision, and headache, with no serious treatment-related adverse events reported over more than 30,000 treatment days.
The approval of VIZZ marks a major advancement in presbyopia treatment, offering a convenient alternative to reading glasses or surgery. It is expected to become broadly available by the fourth quarter of 2025, with possible sample availability as early as October 2025.