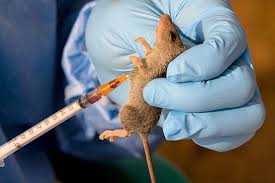
The Coalition for Epidemic Preparedness Innovations (CEPI), says studies are underway to conduct clinical trials to develop a vaccine for Lassa fever in West Africa.
The coalition’s National Project Coordinator for Enable 1.5 in Nigeria, Dr Elsie Ilori, disclosed this on Monday in Abuja at the inauguration of the project.
Lassa fever is a deadly hemorrhagic fever prevalent in West Africa.
According to Ilori, the main purpose of the trials is for clinical studies to be able to produce a vaccine towards Lassa fever disease, to ensure that the burden of the disease in the country and the West Africa region is reduced.
“The study will help us understand Lassa fever itself, how people react to the disease, and the effects of the vaccine on people.
“By understanding the disease, we will be able to understand how the vaccine will work with people.”
She also said that the study aimed to understand Lassa fever’s symptoms and effects on people, evaluate the vaccine’s safety, tolerability, and immunogenicity, and develop a vaccine effective against multiple strains of the disease.
“We are working closely with community leaders, healthcare workers, and local authorities to sensitise the public about the study.
“This includes explaining the study’s objectives, benefits, and potential risks.”
She said that three countries, Nigeria, Sierra Leone, and Liberia were participating, with five sites selected based on high Lassa fever burden.
However, Nigeria has three sites: Federal Medical Centre, Owo, Irrua Specialist Teaching Hospital, Edo state and Alex Ekwueme University Teaching Hospital, Abakaliki.
“These sites were selected due to their high disease burden, increasing the likelihood of enrolling participants with Lassa fever,” Ilori added.
She said that the study prioritised community engagement and sensitisation, whereby researchers would conduct house-to-house enrollment, obtaining consent from household heads.
She also added that participants must provide informed consent and could withdraw at any time without consequences.
She further said that the study would help sensitise people and prepare them for vaccine acceptance.
“The study’s success relies on collaboration between researchers, healthcare workers, and community leaders from Nigeria, Sierra Leone, and Liberia.
“We believe that this study will pave the way for a vaccine, bringing relief to communities ravaged by the disease.
“We are one step closer to a vaccine, and that’s a remarkable achievement.
“The study’s findings is expected to also inform public health policy and guide future vaccine development.”
In addition to CEPI, the study is supported by global health partners, including the World Health Organisation and the Federal Government.
Royce Fulton, the Programme Manager, CEPI for the Enable Lassa Research Programme, said that beyond developing the vaccines through clinical trials, it was important to also be able to identify where the disease was.
Also, to build the necessary capacities within the countries and the partners in Nigeria and other countries in order to be able to really prepare for the great work that lay ahead.
According to him, having clinical trials at a large scale that allows for the identification of the effectiveness of a new vaccine will be with the ambition of having the vaccine ready by 2030 in Nigeria.
“So this Enable 1.5 study, what is going to be the methodology, the outcomes, and when is it possible to be ready? We’re thinking that for the vaccine trials, these are going to be happening imminently.
“We do feel that there is a lot yet to be done for preparedness, for readiness, to make sure the sites and the countries are really equipped with the knowledge, expertise and the tools to be able to conduct large-scale clinical trials.
“So what we have done is spent the past five years implementing a large multi-center prospective cohort trial in order to be able to understand where the disease lies by following 23,000 subjects in the past few years.
“To understand how many of them developed Lassa fever in that time, how many of them are exposed to the vector, which is the rats that live in the houses with them.
“Also, how many of these people can be pulled into further research to help us understand just how effective this vaccine is going to be.”
Participants from Nigeria, Liberia, and Sierra Leone will be enrolled with selection criteria based on age, with adults, adolescents, and children as young as two years old being eligible, as well as people living with HIV.
NAN